Specific gravity
Specific gravity measurements are one of the physical examinations performed during a routine urinalysis.
Urine specific gravity is defined as the density (or weight) of the urine specimen as compared to the density (weight) of distilled water at the same temperature.
The density of the urine specimen is dependent on the
amount and size of the dissolved substances present
in the urine specimen.
These include such substances as glucose, proteins, and electrolytes. The kidneys are responsible for selective reabsorption of essential chemicals and fluid after the blood is filtered.
Abnormal specific gravity values may be an early indication of renal dysfunction.
Low specific gravity urine is considered to be dilute with very few dissolved substances, whereas urine with a high specific gravity indicates that the urine is concentrated with increased amounts of dissolved substances.
Specific gravity may be measured in several ways, but there are three methods used most often in laboratories.These include the use of a refractometer, a urinometer, or a reagent dipstick.
1- Refractometer Measurement Technique
An instrument called a refractometer measures the refractive index of a solution.
This is a measurement of the velocity of light in air as compared to the velocity of light in a solution.
The number of dissolved particles present in the solution affect the velocity and the angle at which the light passes through a urine specimen.
Clinical refractometers (see Fig. ) used for urine analysis use a prism to direct one distinct wavelength of light for comparison to a special scale.
The concentration of the urine specimen has a direct effect on the angle of the light as it passes through to the prism.
Therefractive index is not identical to the specific gravity of urine, but refractometers are calibrated to provide specific gravity readings.
Refractometers are portable, relatively inexpensive, and easily calibrated.
This method of measurement uses only a drop of urine, which can be beneficial as compared to other methods.
Refractometers must be calibrated regularly with distilled water to achieve a result of 1.000.
Five percent NaCl may also be used to check the calibration, and it should read 1.022.
Urine quality control samples should also be used to verify that the refractometer is working correctly and that the operator knows how to read the results appropriately.
Procedure 20-1 provides detailed instructions for the use of a refractometer to measure urine specific gravity.
Procedure 20-1
1. Wash hands
2. Put on gloves.
3. Verify test order and assemble necessary equipment and specimen.
4. Verify specimen labeling and identification.
5. Verify that specimen collection was less than 1 hour before testing or that it was refrigerated and/or preserved appropriately after collection.
6. Allow any refrigerated specimens to come to room temperature before proceeding.
7. Mix specimen well.
8. Pour a small amount of the urine specimen (less than 50 mL) into a transfer tube that has been labeled with the patient’s name and/or ID number.
9. Using distilled water, verify the function of the refractometer:
a. Open the refractometer cover and place one drop of distilled water on the prism under the cover using a clean transfer pipette.
b. Close the cover, and verify that the sample has spread over the entire prism surface.
c. Look at the scale through the eyepiece; bright light in the vicinity helps with visualization.
d. Read the scale where the line intercepts it. This is where the colors change with a sharp line of division.
e. Wipe the sample away from the face of the prism with laboratory tissue and water.
10. Following laboratory protocol, test a quality control specimen using the refractometer. Use the same procedure outlined in step 8 . Chart the results on the quality control log.
11. Using a clean transfer pipette, place one to two drops of the urine specimen (from the transfer tube) on the face of the prism. Following the procedure outlined in step 10 above, read the specific gravity of the specimen.
12. Dispose of the urine left in the transfer tube by pouring it down the sink and flushing it with plenty of water. Dispose of the tube and transfer pipette used for the urine in a biohazard disposal bag. If the urine is not to undergo additional testing immediately, refrigerate it or preserve it accordingly.
13. Clean the instrument using distilled water and a Kimwipe.
14. Remove gloves and sanitize hands.
15. Document results for patient specimen on the chart (if available) and testing log sheet. Be certain to use the correct digits for the documentation.
Because urine specific gravity is measured against distilled water, the results are reported using units that reflect this comparison.Urine specific gravity is reported in units beginning with 1.000 (for distilled water) to amounts above 1.030 (for highly concentrated urine specimens). Urine concentration (as measured by the specific gravity of the urine specimen) may be elevated with congestive heart failure, dehydration, adrenal gland dysfunction, glycosuria (the presence of glucose in the urine), high levels of urine protein, or recent use of high molecular weight IV fluids. The urine specific gravity may also be elevated in situations in which the patient has become dehydrated as a result of excessive vomiting or diarrhea. Decreased urine specific gravity may be present in diabetes insipidus, excessive fluid intake, or renal failure. The normal urine specific gravity is 1.005 to 1.030
2-Urinometer Measurement Technique
A urinometer is a device that uses a weighted float attached to a scale.
This weighted float will displace a volume of liquid relative to its weight. When used with distilled water, the weight will sink until the calibrated scale reads exactly 1.000.
Because urine has more dissolved substances present than does distilled water, the urinometer float will displace less fluid.
This will result in a higher reading on the urinometer scale. Figure 20-4 includes an example of a urinometer.
Urinometers are rarely used for specific gravity testing.
The Clinical and Laboratory Standards Institute does not recommend their use for urine specific gravity, but they may still be used in small laboratories.
There are several disadvantages to the urinometer method, including the large volume of urine required for the procedure (10 to 15 mL), the fragilityof the weighted float/scale, and the need for a specific container that allows for the displacement of the fluid during the process.
In addition, the specific gravity is measured at the meniscus (the bottom of the curve at the top of the urine column) of the fluid after the float is added to the urine specimen; the reported results may sometimes be inaccurate because of problems with reading the meniscus properly.
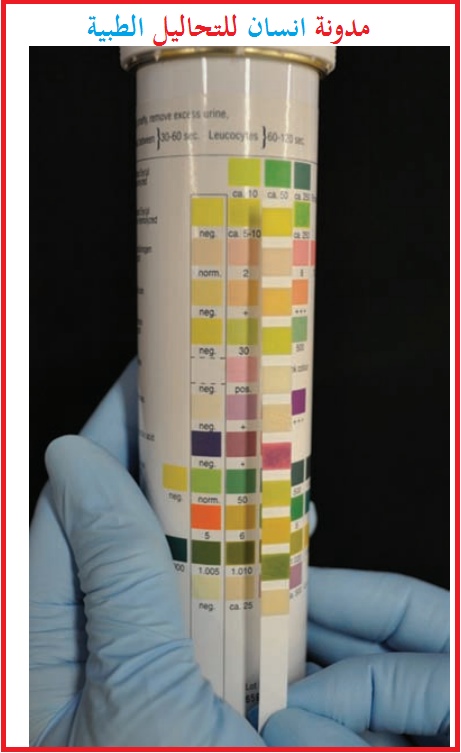
3-Reagent Strip Methodology
Specific gravity measurements performed by using reagent strips are another indirect measurement of the amount of dissolved substances in the urine specimen.
Reagent strip reactions are based on the release of ionic solutes in the specimen.
These ions are released formeasurement when exposed to thereagent pad on thestrip as it is dipped into the urine specimen.
A colorchange occurs on the reagent pads, which can be measuredin 0.005 intervals from 1.000 to 1.030.
Many facilities have adopted this method of measuring specific gravity because it does not involve an additional instrument for the procedure, as the results are read for specific gravity while reading those for other chemical analytes on the reagent strip.
Reference ranges for urine
specific gravity are approximately 1.005 to 1.030.
د|على العربى










ليست هناك تعليقات:
إرسال تعليق