EXPERIMENT ON DETERMINATION OF CONDUCTIVITY
PREAMBLE:
“How to determine conductivity in Water and Wastewater”.Test procedure is in accordance to IS: 3025 (Part 14) - Reaffirmed 2002. In addition to our Indian Standard, we also discuss in brief regarding the procedure stated in
(1) APHA Standard Methods for the Examination of Water and Wastewater - 20th Edition. Method 2510.
(2) Methods for Chemical Analysis of Water and Wastes, EPA-600/4-79 020, USEPA, Method 120.1.
AIM
To determine the conductivity of given water sample with the stipulations as per IS: 3025 (Part 14) - Reaffirmed 2002.
INTRODUCTION
Conductivity of a substance is defined as 'the ability or power to conduct or transmit heat, electricity or sound'. When an electrical potential difference is placed across a conductor, its movable charges flow, giving rise to an electric current. This property is called conductivity. Since the charge on ions in solution facilitates the conductance of electrical current, the conductivity of a solution is proportional to its ion concentration.
The electrical conductivity can be expressed as mhos (Reciprocal of ohms) or as siemens. The conductivity of water is a measure of the ability of water to carry an electric current. In most water, the conductivity is very low, so millisiemens or microsiemens are used as units for water conductivity. The conductivity of water is directly linked to the concentration of the ions and their mobility. The ions in water acts as electrolytes and conducts the electricity.
The conductivity depends on the value of the pH, on the temperature of measurement and on the amount of CO2 which has been dissolved in the water to form ions. The conductivity is also affected by the concentration of ions already present in the water such as chloride, sodium and ammonium. Chemical composition of water determines its conductivity. Hence this becomes the most widely used measure of the purity of water.
ENVIRONMENTAL SIGNIFICANCE
• Electrical conductivity measurements are often employed to monitor desalination plants.
• It is useful to assess the source of pollution.
• In coastal regions, conductivity data can be used to decide the extent of intrusion of sea water into ground water.
• Conductivity data is useful in determining the suitability of water and wastewater for disposal on land. Irrigation waters up to 2 millisiemens / cm conductance have been found to be suitable for irrigation depending on soils and climatic characteristics.
• It is also used indirectly to fine out inorganic dissolved solids.
• It is useful to assess the source of pollution.
• In coastal regions, conductivity data can be used to decide the extent of intrusion of sea water into ground water.
• Conductivity data is useful in determining the suitability of water and wastewater for disposal on land. Irrigation waters up to 2 millisiemens / cm conductance have been found to be suitable for irrigation depending on soils and climatic characteristics.
• It is also used indirectly to fine out inorganic dissolved solids.
PRINCIPLE
Conductivity is measured with a probe and a meter. A voltage is applied between the two electrodes in the probe immersed in the sample water. The drop in voltage caused by the resistance of the water is used to calculate the conductivity per centimeter. Conductivity (G), the inverse of resistivity (R) is determined from the voltage and current values according to Ohm’s law. i.e. R=V/I then, G=1/R=I/V. The meter converts the probe measurement to micro mhos per centimeter and displays the result for the user.
MATERIALS REQUIRED
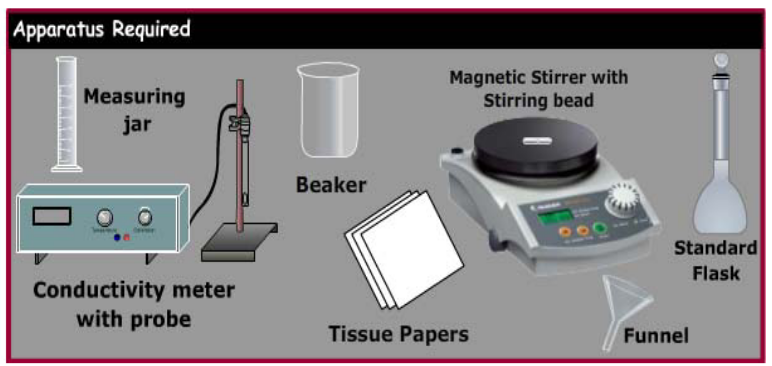
APPARATUS REQUIRED
1. Conductivity Meter with Electrode /ATC probe
2. Magnetic Stirrer with stirring bead
3. Standard flask
4. Measuring jar
5. Beaker 250 mL
6. Funnel
7. Tissue Paper
2. Magnetic Stirrer with stirring bead
3. Standard flask
4. Measuring jar
5. Beaker 250 mL
6. Funnel
7. Tissue Paper
CHEMICALS REQUIRED
1. Potassium Chloride
2. Distilled Water
2. Distilled Water
SAMPLE HANDLING AND PRESERVATION
Water samples should be collected in plastic cans or glass bottles. All bottles must be cleaned thoroughly in phosphate-free detergent and rinsed thoroughly with both tap and distilled water.
Volume collected should be sufficient to insure a representative sample, allow for replicate analysis (if required), and minimize waste disposal.
Analysis should begin as soon as possible after the collection. If the analysis is not completed within 12 hours of sample collection, sample should be filtered through a 0.45μ filter paper and stored at 4°C. High quality distilled water must be used for washing the filter and apparatus and needs to be rinsed with sample before use.
No chemical preservation is required. Keep the samples at 4°C. DO NOT ALLOW SAMPLES TO FREEZE.
PRECAUTIONS
The following precautions should be observed while performing the experiment:
1. Switch on the conductivity meter for atleast 30 minutes before starting the experiment so that the instrument gets stabilizes.
2. As it involves instruments for analyzing do not forget to calibrate the instrument.
3. Always prepare the calibration solution freshly before the st art of the experiment.
4. As conductance is influenced by temperature, always use a conductivity meter with temperature control.
5. Always dip the electrode in distilled water and do not expose it to air.
1. Switch on the conductivity meter for atleast 30 minutes before starting the experiment so that the instrument gets stabilizes.
2. As it involves instruments for analyzing do not forget to calibrate the instrument.
3. Always prepare the calibration solution freshly before the st art of the experiment.
4. As conductance is influenced by temperature, always use a conductivity meter with temperature control.
5. Always dip the electrode in distilled water and do not expose it to air.
PROCEDURE
For testing the given water sample first the reagents are to be prepared. Then the Conductivity Meter is required to be calibrated.
PREPARATION OF REAGENTS
Potassium Chloride Solution (0.1N):
• Switch on the Electronic balance, keep the weighing pan, set the reading to zero.• Measure 50 mL of distilled water and transfer it to the beaker.
• Weigh 0.7456g of Potassium chloride.
• Transfer the 0.7456g of potassium chloride to the beaker contains distilled water and mix it by the glass rod until it dissolves thoroughly.
• Transfer the contents to the 100 mL standard flask.
• Make up the volume to 100 mL, by adding distilled water and shake the contents well. This solution is used to calibrate the conductivity meter.
• Switch on the Electronic balance, keep the weighing pan, set the reading to zero.
• Weigh 0.7456g of Potassium chloride.
• Transfer the 0.7456g of potassium chloride to the beaker contains distilled water and mix it by the glass rod until it dissolves thoroughly.
• Transfer the contents to the 100 mL standard flask.
• Make up the volume to 100 mL, by adding distilled water and shake the contents well.
An overview on conductivity meter:
An electrical conductivity meter is used to measure the conductivity in a solution. Basically the conductivity unit consists of 1. Conductivity Meter
2. Electrode / ATC probe
3. Magnetic stirrer with bead
Before starting the experiment, switch on the instrument for atleast 30 minutes, so that the instrument stabilizes. Using the same electrode the Conductivity meter can measure three parameters
1. Conductivity
2. Selenity
3. Total Dissolved Solids (TDS)
The Room temperature can also be displayed using Automatic temperature control (ATC) probe. The Mode button is pressed to select the parameter to be measured. The Display can show conductivity reading in microsiemens or in millisiemens. The Conductivity of a solution is highly influenced by temperature therefore it is necessary to calibrate the instrument at the same temperature as the solution is being measured.
2. Electrode / ATC probe
3. Magnetic stirrer with bead
1. Conductivity
2. Selenity
3. Total Dissolved Solids (TDS)
Calibration of Conductivity Meter
Take 0.1N Potassium Chloride in a beaker. Switch on the magnetic stirrer and place the beaker on the stirrer. Insert the magnetic bead in the beaker. Place the electrode inside the solution. Select the calibration button and using up and down key adjust the conductivity of the 0.1N potassium chloride solution to 14.12 millisiemens / cm at 30oc. Now the meter is ready for the measurement of samples. TESTING OF WATER SAMPLE
• Rinse the electrode thoroughly with deionised water and carefully wipe with a tissue paper.
• Measure 200 mL of water sample and transfer it to a beaker and place it on the magnetic stirrer.
• Dip the electrode into the sample solution taken in a beaker and wait for a steady reading. Make sure that the instrument is giving stable reading.
• Note down the reading in the display directly, which is expressed in millisiemens. The reading is 5.97 millisiemens.
-----------
Water Analysis-Determination of Chemical Parameters
Take 0.1N Potassium Chloride in a beaker.
Switch on the magnetic stirrer and place the beaker on the stirrer.
Insert the magnetic bead in the beaker.
Place the electrode inside the solution.
Select the calibration button and using up and down key adjust the conductivity of the 0.1N potassium chloride solution to 14.12 millisiemens / cm at 30oc.
Now the meter is ready for the measurement of samples.
TESTING OF WATER SAMPLE
• Rinse the electrode thoroughly with deionised water and carefully wipe with a tissue paper.• Measure 200 mL of water sample and transfer it to a beaker and place it on the magnetic stirrer.
• Dip the electrode into the sample solution taken in a beaker and wait for a steady reading. Make sure that the instrument is giving stable reading.
• Note down the reading in the display directly, which is expressed in millisiemens. The reading is 5.97 millisiemens.
-----------
Water Analysis-Determination of Chemical Parameters











ليست هناك تعليقات:
إرسال تعليق